
Free ISO 13485 Audit Checklists PDF SafetyCulture
In this document, you will find an explanation of which documents are mandatory according to the ISO 13485:2016 standard, and which non-mandatory documents are commonly used in the QMS implementation, in the same order and numbered clauses as in ISO 13485. Introduction

ISO 13485 and 21 CFR Part 820 Internal Audit and Gap Analysis Checklist ZenonHost Easy
This checklist is based on the information provided in the 2016-03-01 release of the ISO 13485:2016 international standard. The checklist is best used by trained and practicing auditors to evaluate or assess Quality Management Systems requirements based on the standard.
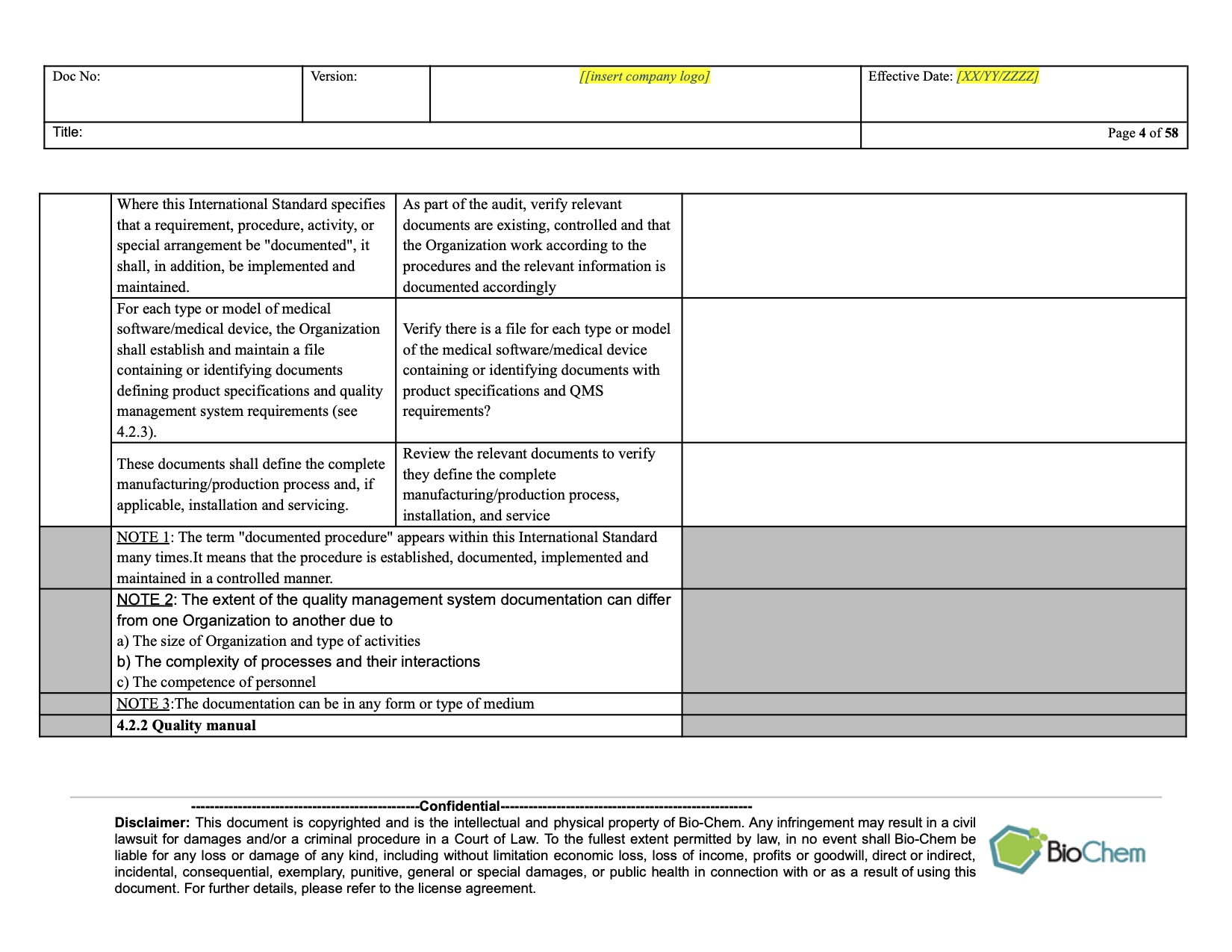
ISO 13485 Internal Audit Planning and Scheduling BioChem
ISO 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. Such organizations can be involved in one or more stages of the life-cycle, including design and.
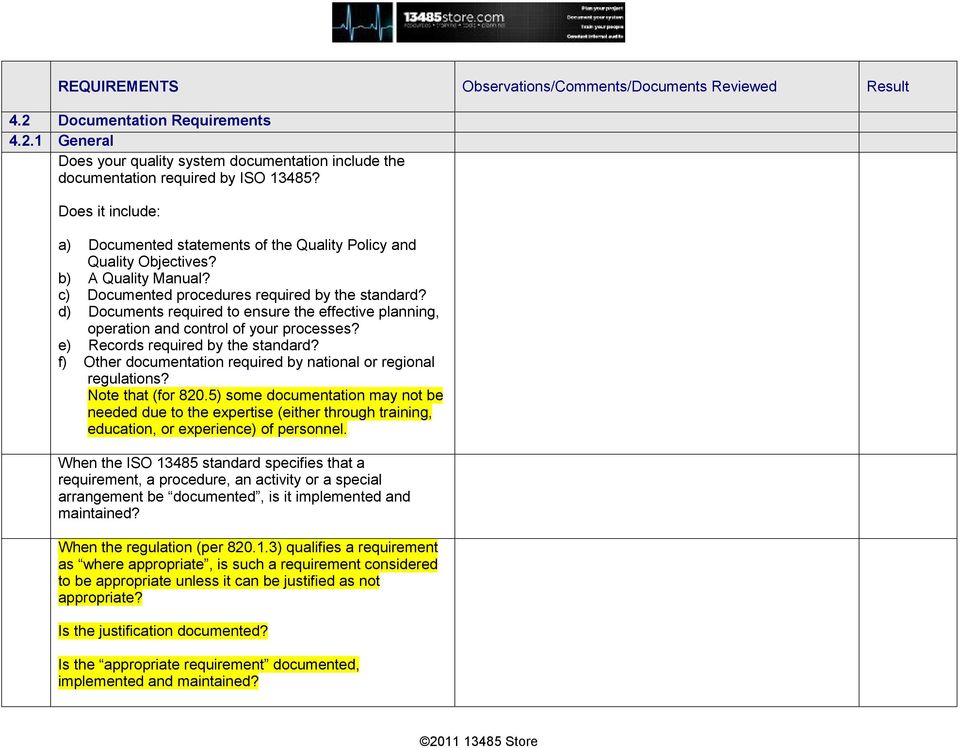
Iso 13485 Internal Audit Checklist interactivelasopa
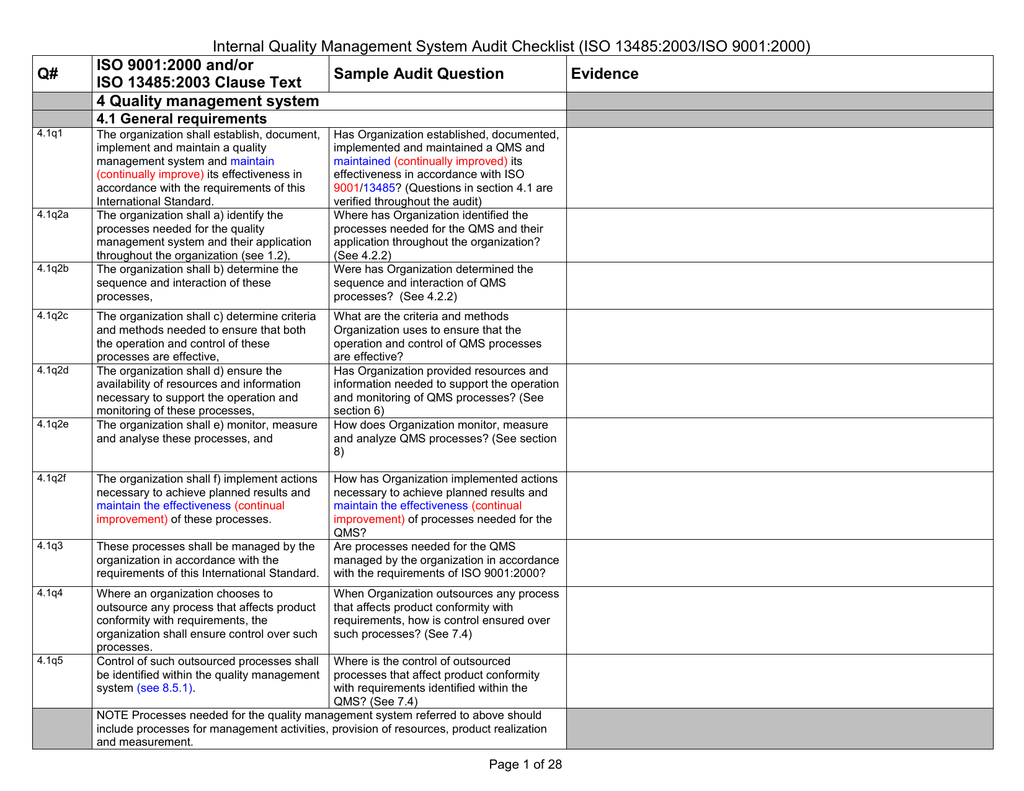
ISO 13485 compliance checklist Documentation requirements Quality manual Medical device file General 4.2 4.2.2 4.2.3 4.2.1 Does the quality management system documentation (see 4.2.4) include: a) the scope of the quality management system, including details of and justification for any exclusion or non-application?

Audit Checklist for ISO 13485 Audit Technology
The most effective method to comply with ISO 13485 is establishing a QMS and using an audit checklist to aid in certification. A digital QMS tracks, manages, and organizes all internal processes; and can be integrated with existing systems and solutions.

ISO 134852003 Audit Check List
The Internal Audit Checklist is the list of questions required to ensure the management system is implemented and maintained. The listing includes more than 100 questions to ensure each requirement of the ISO 13485 standard is implemented and maintained within the Quality Management System, and includes the ability for the company to add additional questions to suit individual needs.

ISO 13485 Documentation Requirements
The ISO 13485 audit checklist is used for internal audit while preparing the system for a third-party ISO 13485 certification audit. The ISO 13485 audit checklist allows quality managers to document evidence of compliance based on processes, standard requirements, and process characteristics.
Contoh Matriks Kompetensi Karyawan IMAGESEE
ISO 13485 audit requirements. The objective of ISO 13485 audits is to determine if all applicable requirements of ISO 13485:2016 have been implemented in your company. The audit objectives specifically include evaluation of: The effectiveness of your QMS in incorporating the applicable regulatory requirements. Product/process-related technologies.
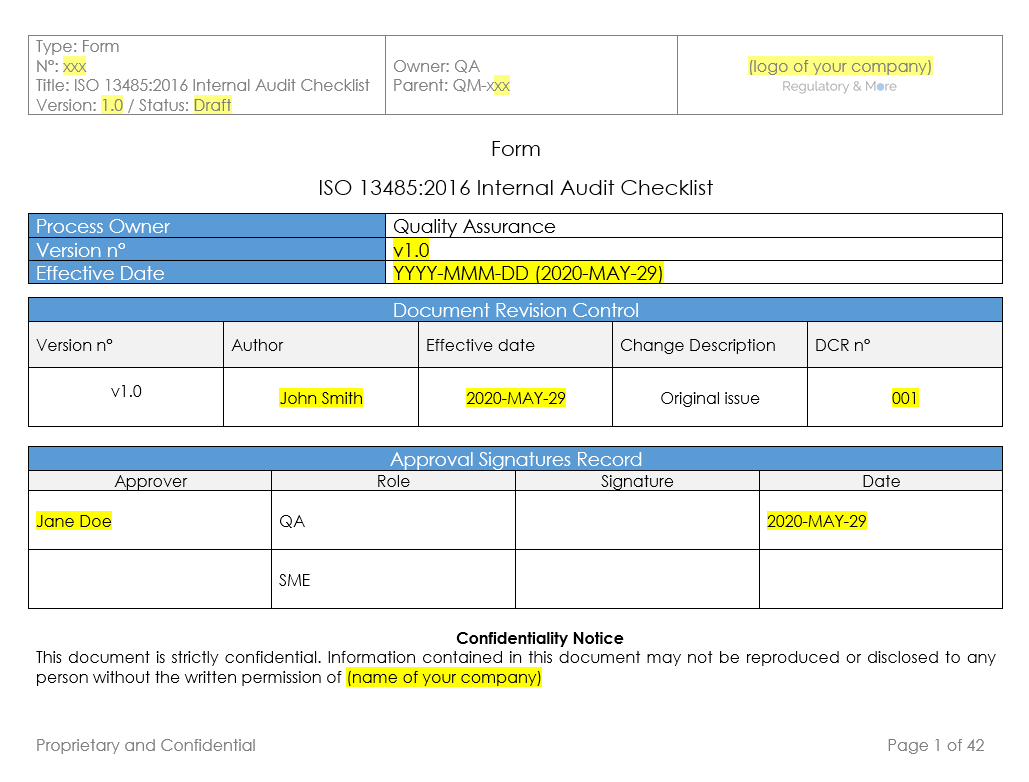
Checklist ISO 13485 2016 Internal Audit (v.1.0) Regulatory and More
This third edition of ISO 13485 cancels and replaces the second edition (ISO 13485:2003) and ISO/TR 14969:2004, which have been technically revised. It also incorporates the Technical Corrigendum ISO 13485:2003/Cor.1:2009. A summary of the changes incorporated into this edition compared with the previous edition is given in Annex A.
Quality Management System Audit Checklist Gambaran
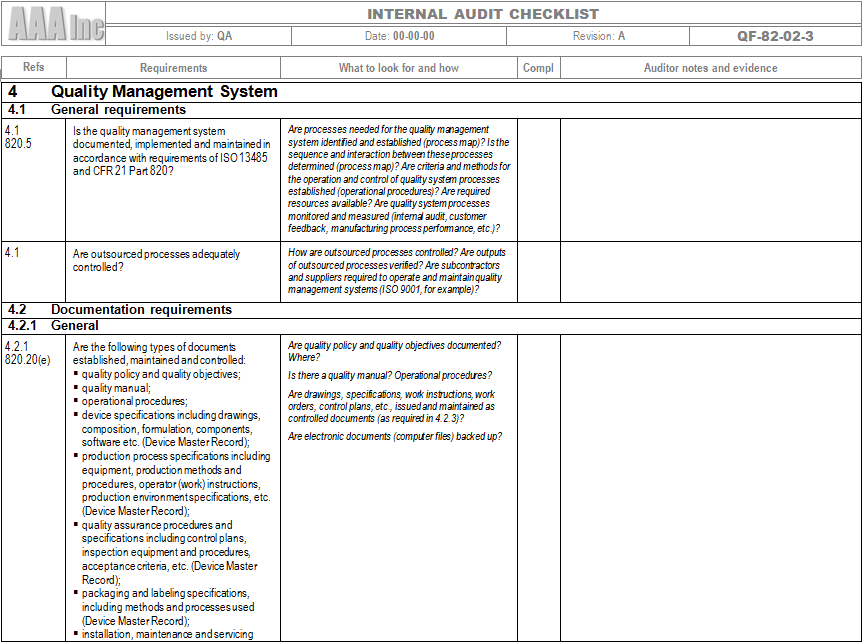
An ISO 13485 audit checklist is utilized by quality managers to determine if the organization's QMS is aligned with the ISO 13485:2016 standard. It helps evaluate an organization's readiness for a third-party ISO 13485:2016 certification audit.
Iso 13485 Audit Checklist passlbob
The ISO 13485 audit checklist identifies the key areas that medical device companies must address to ensure compliance with the regulation and maintain patient safety. The checklist can be used to conduct a comprehensive review of the quality management system and identify areas for improvement.
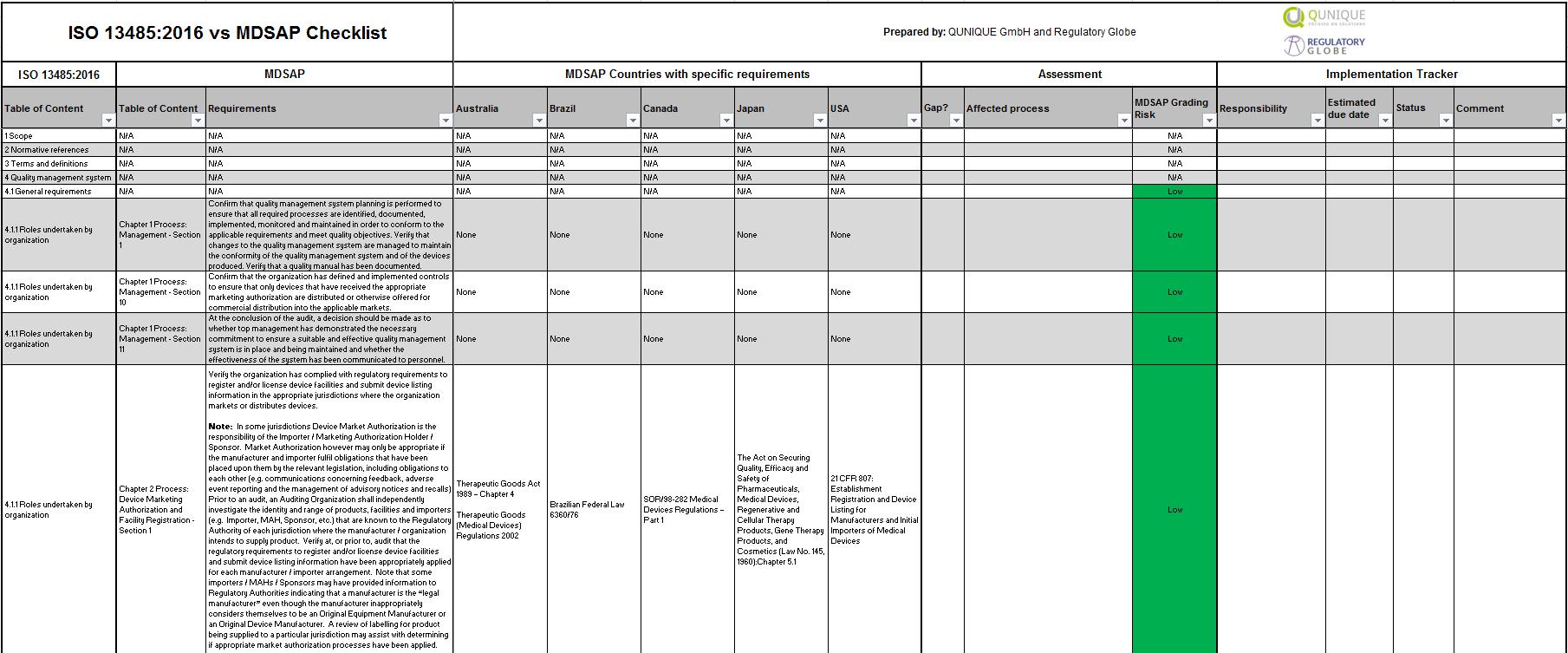
MDSAP vs. ISO 134852016 Checklist Regulatory Globe
Checklist for ISO 13485 implementation Once you understand why to implement 13485, you can follow this checklist of ISO 13485 implementation steps to get started with the project. Diagram of the ISO 13485:2016 Implementation Process Free diagram that outlines the steps for your ISO 13485 implementation Download now 1) Get management support
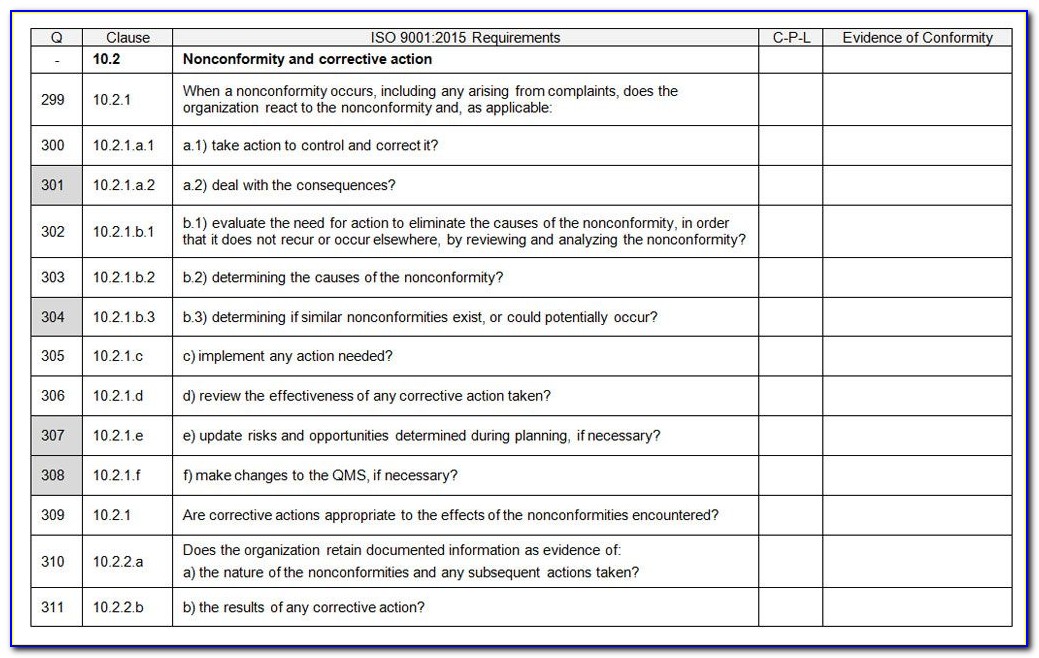
Iso 13485 Audit Checklist Template 2023 Template Printable
Forms and Checklists: Risk Management Templates: Document Control Templates: Training Records: Internal Audit Templates: Supplier Evaluation and Control Templates: Conclusion ISO 13485 is an international standard for quality management systems (QMS) specifically designed for the medical device industry.
Iso 13485 audit checklist
An ISO 13485 audit checklist is utilized by quality managers to determine if the organization's QMS is aligned with the ISO 13485:2016 standard. It helps evaluate an organization's readiness for a third-party ISO 13485:2016 certification audit. With SafetyCulture (formerly iAuditor), quality managers can:
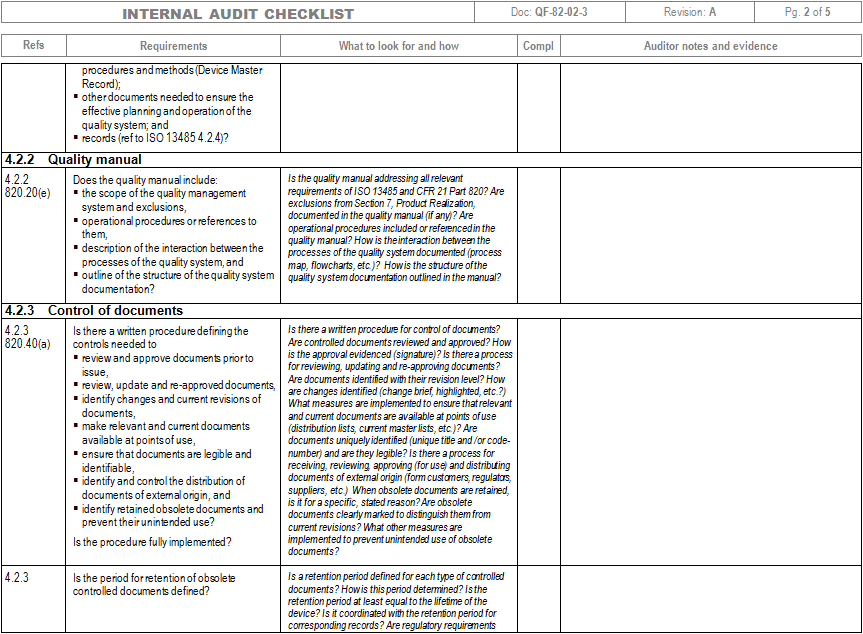
ISO 13485 and 21 CFR Part 820 Internal Audit and Gap Analysis Checklist ZenonHost Easy
Download now How do you create a checklist to check conformance? An internal audit is there to witness the outcome of a process through a review of records or witnessing the actions of the employees, and then to compare this to the planned arrangements for the process to see if what is being done is what was planned.

ISO 13485 2016 Internal Auditor Checklist TQS Inc.
ISO 13485:2016 - Medical devices - A practical guide has been authored by technical experts of ISO/TC 210. The handbook is intended to guide organizations in the development, implementation and maintenance of their quality management system in accordance with ISO 13485. Organizations active in the medical device sector, such as.